Clinical and commercial
Working with partners to evolve our technology and reach more underserved populations

A breast exam with Bexa is a highly accurate means of identifying abnormal breast masses, the most common presentation of invasive breast cancer
Clinical Overview
Bexa represents a significant advancement in breast cancer screening technology, offering a highly accurate method for identifying abnormal breast masses, the most common presentation of invasive breast cancer. As a Class II FDA-cleared (K181672) medical device, Bexa is indicated for the documentation of abnormal breast tissues and masses discovered during breast examination.
Key Clinical Features
- High Sensitivity: Breast examinations with Bexa demonstrate a sensitivity of 90% in mapping breast masses as small as 5mm.
- Density-Independent: Bexa’s pressure-based elastography is unaffected by breast density, ensuring consistent accuracy across all tissue types.
- Low False Positive Rate: The technology’s specificity reduces the incidence of false positive results, minimizing unnecessary follow-up procedures.
- Effective in Younger Populations: Bexa maintains accuracy in younger women who fall outside USPSTF guidelines for mammography, addressing a critical gap in current screening protocols.
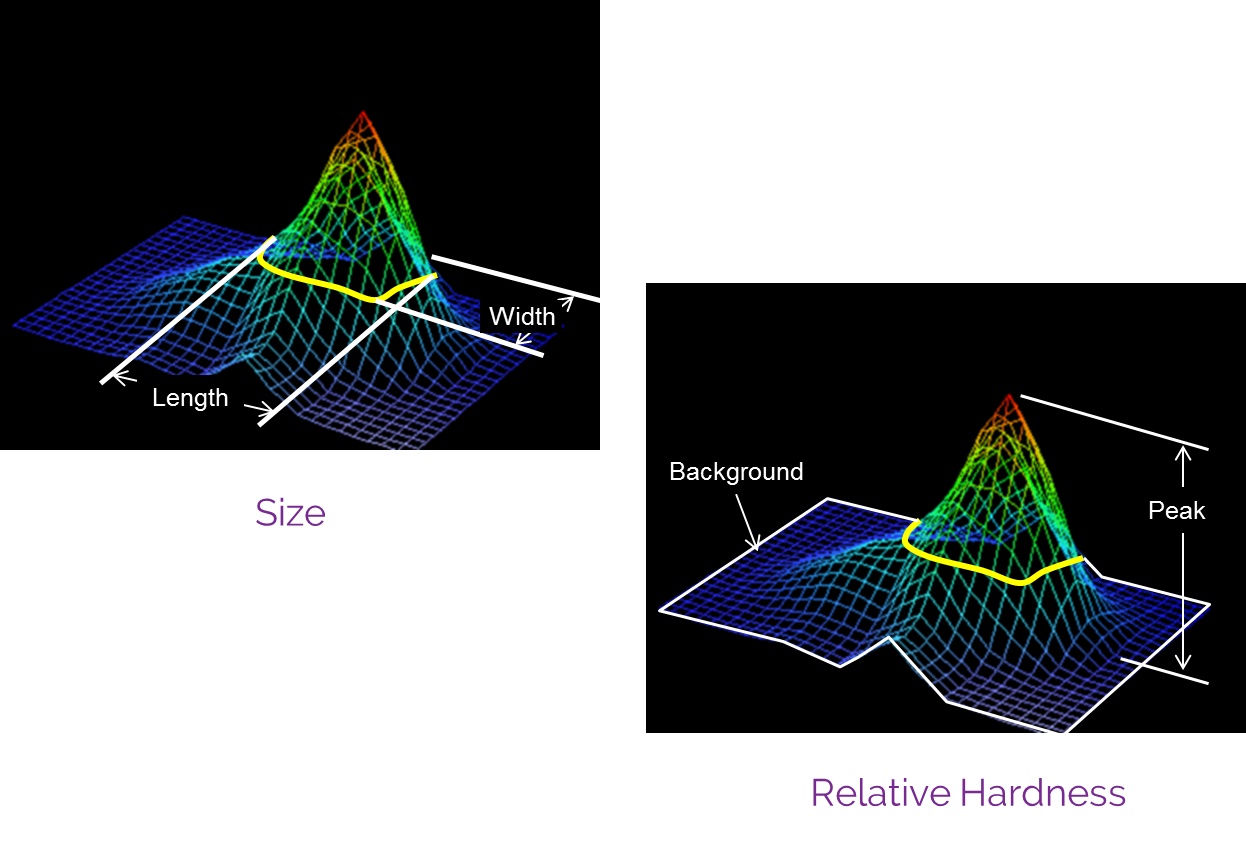
The technology
Bexa employs high-resolution elastography to produce a detailed map of abnormal breast tissues and masses. The device’s proprietary capacitive sensor array collects data on tissue elasticity, capturing differences in firmness compared to surrounding tissue. This non-invasive approach involves no radiation emission, offering a safe alternative to traditional imaging methods.
Integrated Ultrasound
A Bexa examination fully integrates B-mode ultrasound evaluation of all discovered breast masses. This comprehensive approach adds critical data to the evaluation and classification of abnormal breast tissues, significantly reducing the need for additional imaging studies.
Clinical Validation
Multiple U.S. clinical studies, both completed and nearing completion, demonstrate the efficacy of Bexa in discovering abnormal masses. These studies not only validate the technology’s clinical capabilities but also highlight its potential to drive social change and economic impact through consistently high adoption rates among women.
Patient Experience
Bexa examinations are designed to be quick, painless, and radiation-free, providing immediate results. This patient-centric approach addresses many of the barriers associated with traditional breast cancer screening methods, potentially increasing compliance with regular screening recommendations.
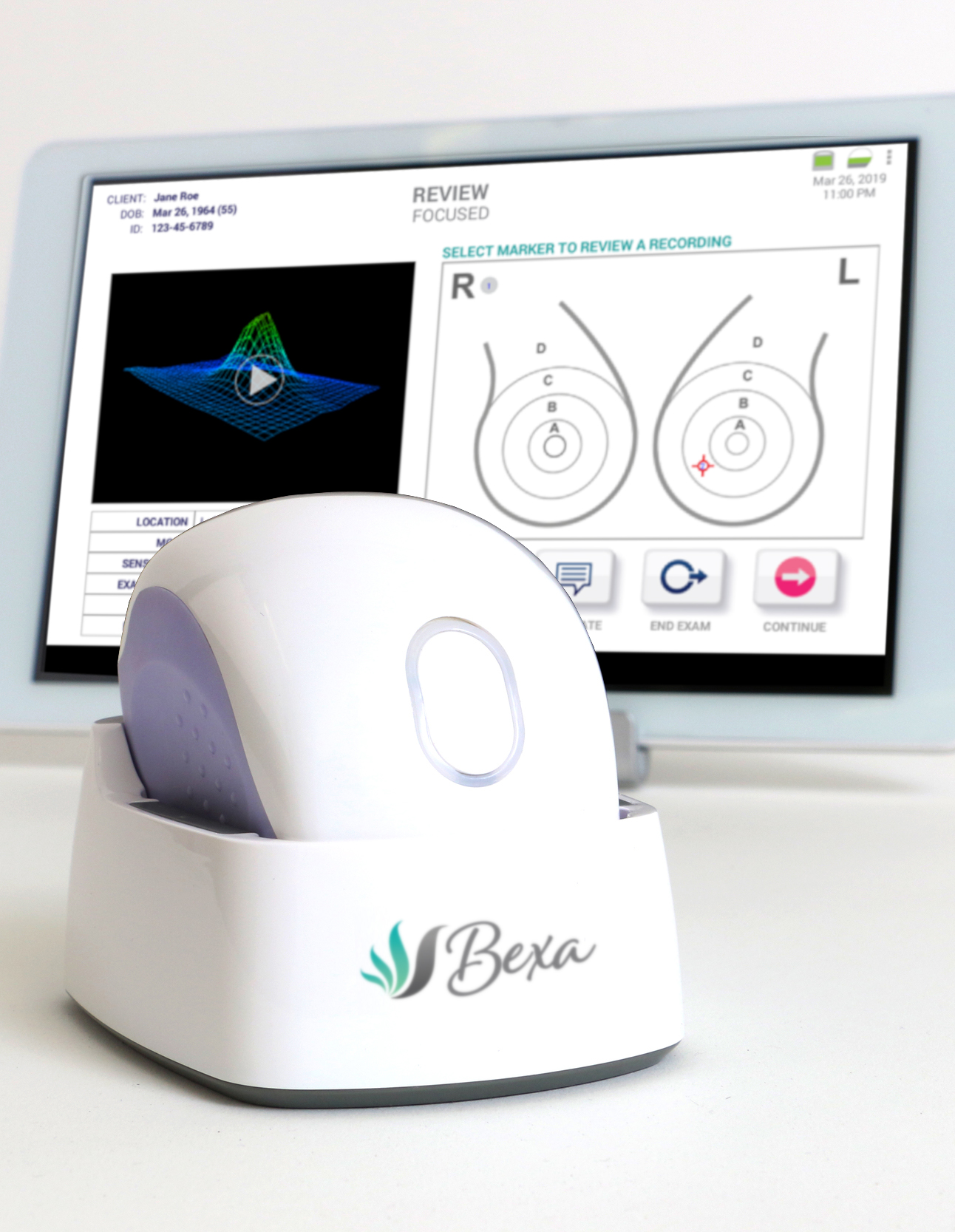
Who owns Bexa?
Sure, Inc., (“Sure”) is a physician-led company that develops, owns all intellectual property related to, and manufactures the Bexa device.
Sure, operates from bases in Los Angeles, New York, Dubai (UAE), Amman (Jordan) and Bogota (Colombia).
We provide training in the use of the Bexa device and own and operate the SureView system, as well as operating a continuous R&D and AI development activity.

Implications for Clinical Practice
- Enhanced Early Detection: Bexa’s high sensitivity for small masses may lead to earlier detection of breast cancer, potentially improving treatment outcomes.
- Reduced Unnecessary Procedures: The low false-positive rate may decrease the number of unnecessary biopsies and follow-up imaging studies.
- Expanded Screening Population: Efficacy in younger women and those with dense breast tissue allows for more comprehensive screening across diverse patient populations.
- Improved Patient Compliance: The non-invasive, pain-free nature of Bexa examinations may encourage more regular screening among patients hesitant about traditional mammography.
For more information on integrating Bexa into your clinical practice or to review detailed clinical study results, please contact our medical liaison team.
Thank you for taking the time to learn about Bexa
We’re working hard to continuously advance the sophistication of Bexa and to establish its role in the early detection of breast cancer.